Kymeria (tisagenlecleucel), developed by Novartis and approved by the European Medicines Agency (EMA), is a CAR-T cell therapy used to treat acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL). Its effectiveness is remarkable—but so is the price.
Understanding the true cost of treatment in Europe, and the factors behind it, is essential for patients exploring their options.

Photo: #CART25 - European hematology conference in Strasbourg, France.
What Is the Cost of Kymeria in Europe in 2025?
In 2025, the list price of Kymriah (Kymeria is the international brand name, also spelled Kymriah in many sources) varies by country, due to differences in healthcare systems and negotiated reimbursement agreements.
Approximate prices by country (public health system rates):
- Germany: €320,000–€350,000 per treatment
- France: €330,000–€340,000
- Italy: €300,000–€320,000 vUK (NHS England): Negotiated confidential discount from the list price of £282,000, but estimated effective cost is £250,000–£270,000
- Spain & Netherlands: Between €300,000 and €350,000, based on outcome-based payment models
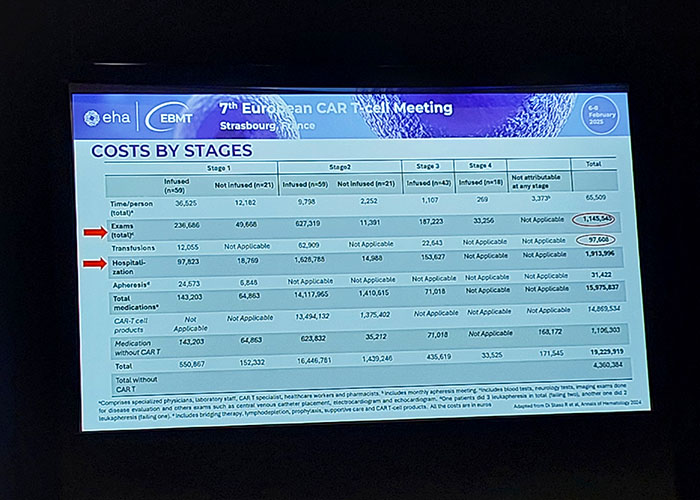
Photo: #CART25 - European hematology conference in Strasbourg, France.
What’s Included in the Cost?
The full cost of Kymeria includes more than just the CAR-T infusion. Other costs may include:
- Cell collection and manufacturing (personalized T-cell modification)
- Hospital stay and monitoring
- Pre-treatment chemotherapy
- Side effect management, especially for cytokine release syndrome (CRS)
- Follow-up care
When factoring in these additional services, the total cost of CAR-T therapy can reach €400,000–€500,000 per patient in Western Europe.
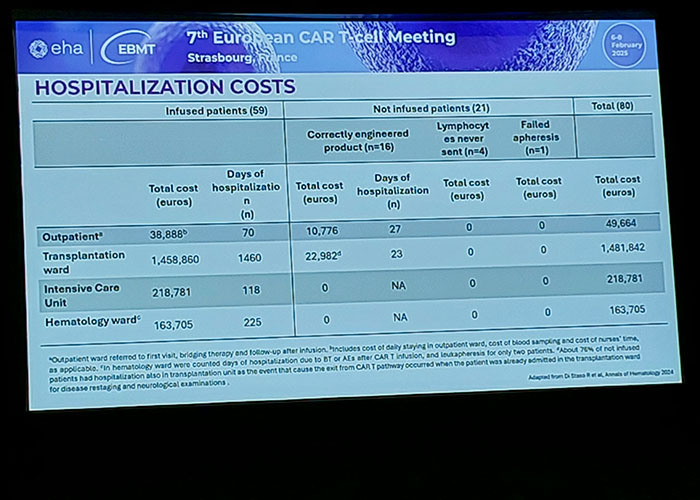
Photo: #CART25 - European hematology conference in Strasbourg, France.
Why Are Prices So High?
- Complexity of manufacturing: Each dose is custom-made for the individual using their own T cells.
- Limited centers: Only certified hospitals can administer Kymeria due to its complexity and safety requirements.
A More Affordable Alternative: CAR-T Therapy in Israel
Israel has emerged as a world leader in CAR-T therapy, offering Western-standard care at 30%–50% lower costs, with no compromise in medical quality or experience.
Publication date: March 2025
Sources
Novartis. Kymriah (tisagenlecleucel) EU product information.
NICE UK. Kymriah appraisal and reimbursement.
EMA approval reports and pricing insights – Evaluate Pharma database (2023–2025 trends)
Health system publications from Germany, France, and Italy (2024–2025 pricing data)