Chimeric Antigen Receptor T-cell (CAR-T) therapy is an innovative treatment that has shown significant promise for patients with multiple myeloma (MM), especially for those who have exhausted conventional treatment options. This article explains who may qualify for CAR-T therapy, when it’s typically recommended, and key developments in this field.
What Is CAR-T Therapy?
CAR-T therapy involves collecting a patient’s own T cells, engineering them to recognize specific proteins found on cancer cells, and then reintroducing them into the body to attack the disease. In multiple myeloma, these engineered cells target BCMA (B-cell maturation antigen), which is present on malignant plasma cells.
The FDA has approved two CAR-T treatments for MM: Abecma (idecabtagene vicleucel) and Carvykti (ciltacabtagene autoleucel).
In addition to commercial treatments, several academic centers offer their own CAR-T programs for multiple myeloma, often delivering comparable results at a lower cost.
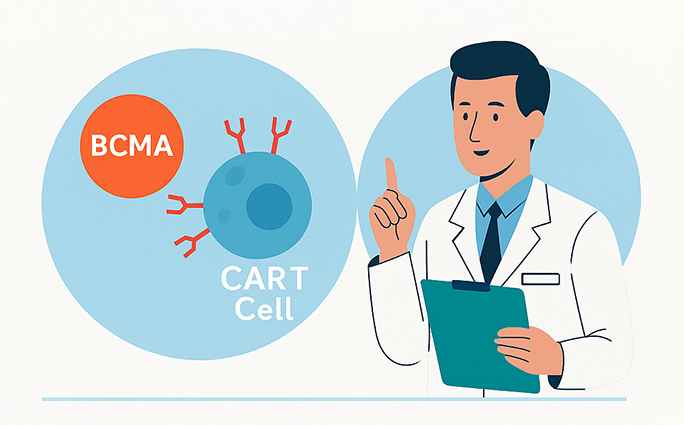
Who Qualifies for CAR-T Therapy for Multiple Myeloma?
Eligibility is based on various clinical and physical factors:
- Disease Progression: Typically, candidates have relapsed or refractory MM and have already undergone 2–3 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody.
- Overall Condition: Patients should be in good physical health—commonly measured with an ECOG performance status of 0–2. In everyday terms, this means the person should be able to care for themselves independently.
- Organ Function: Heart, kidney, liver, and lung functions must be within acceptable ranges, usually confirmed through blood tests.
- Age Limitations: There’s no official age cutoff. Patients as old as 91 have successfully received CAR-T therapy.
- Infection Control: Any active infections must be fully treated before beginning therapy.
Note: Each hospital or clinical trial may have slightly different eligibility guidelines.
When Is the Right Time for CAR-T Therapy?
Doctors usually recommend CAR-T for patients who:
- Have relapsed or difficult-to-treat myeloma after standard treatments
- Are not candidates for stem cell transplantation
- Are in good physical condition with stable organ function
There is growing evidence that introducing CAR-T earlier in the treatment journey may improve outcomes. Several clinical trials are currently testing this approach.
What Are the Risks?
Though CAR-T has changed the outlook for many patients, it does come with some potential side effects:
- Cytokine Release Syndrome (CRS): A strong immune reaction that can lead to fever, low blood pressure, and inflammation.
- Neurological Effects: Temporary confusion, headaches, or seizures can occur in some patients.
These effects are typically managed with medications and close monitoring in specialized centers.
Publication date: May 6, 2025.
Sources
1. American Cancer Society – CAR T-cell Therapy for Multiple Myeloma
2. Massachusetts General Hospital – CAR T-Cell Therapy for Multiple Myeloma
3. CARTHope – Patient Eligibility for CAR-T Therapy
4. National Cancer Institute – FDA Approves Carvykti CAR T-Cell Therapy for Multiple Myeloma
5. NIH / PubMed Central – CAR T-Cell Therapy for Patients with Multiple Myeloma