The 7th European CAR T-cell Meeting, which took place in France in February 2025, addressed several challenges related to accessibility in CAR T-cell therapy:
Economic and Organizational Challenges
- Cost management: The meeting highlighted the significant economic impact of CAR-T therapy and emphasized the need for cost-effective patient management and better resource allocation.
- Flexible reimbursement policies: Discussions focused on the importance of developing flexible reimbursement policies to enhance access to CAR-T therapy.
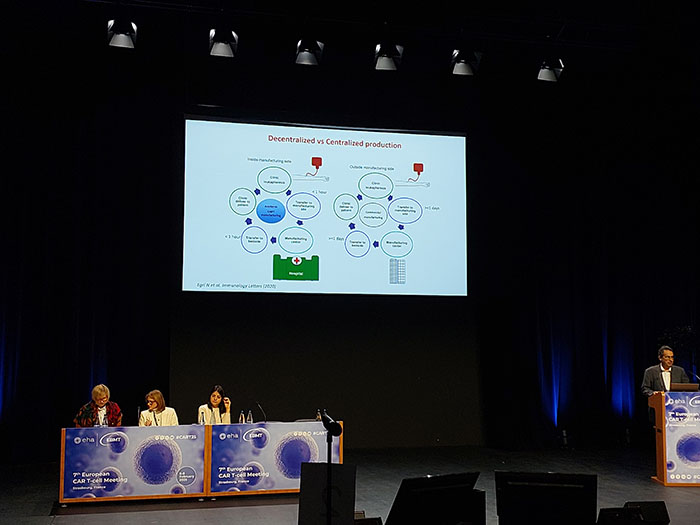
- Decentralized production: Advocates proposed decentralized and academic CAR-T production through hospital exemption as a more personalized and cost-effective approach.
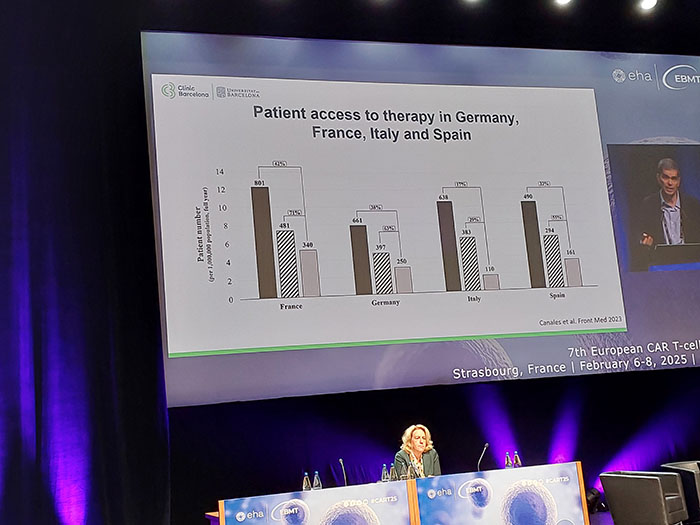
Healthcare System Challenges
- Health system bottlenecks: The meeting addressed the need to improve patient referral and approval processes to enhance access.
- Infrastructure development: Discussions emphasized the importance of developing infrastructure to make CAR-T therapy more widely accessible.
- Multidisciplinary teams: The meeting stressed that CAR-T therapy should be performed at centers with multidisciplinary teams and sufficient infrastructure to manage logistics and patient care.
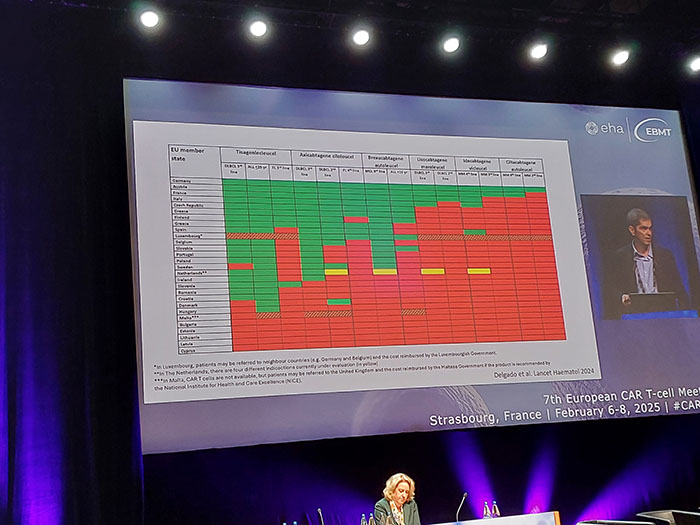
Availability of various CAR-T programs in Europe
Patient-Specific Challenges
- Travel and housing support: The meeting recognized the need to provide travel and housing support for patients who must travel long distances for treatment.
- Caregiver requirements: Discussions acknowledged the burden on patients to have a caregiver during treatment and the need to address this challenge.
- Health disparities: The meeting highlighted how factors such as older age, lower socioeconomic status, and inadequate insurance coverage contribute to limiting access to CAR-T therapies.
Future Directions
- Bringing therapy to patients: The meeting discussed the potential for future developments that would allow providers to bring CAR-T treatment to patients rather than requiring patients to travel to specialized centers.
- Streamlining approval processes: Efforts to streamline the approval process for CAR-T therapy were discussed as a means to improve access.
- Increasing production capacity: The meeting addressed the need to increase production capacity to make CAR-T therapy more widely available.
By addressing these challenges, the meeting aimed to pave the way for more equitable access to CAR-T cell therapy, ensuring that a broader range of patients can benefit from this innovative treatment.
Publication date: March 2025.