Bridging therapy is increasingly used in CAR-T treatment to manage disease progression during manufacturing delays, with commercial CAR-T therapies requiring it more frequently due to longer logistical timelines compared to academic counterparts. This difference stems from variations in production infrastructure and treatment protocols.
Bridging Therapy Utilization
Purpose and Timing:
- Prevents tumor progression during CAR-T manufacturing (typically 2–6 weeks)
- Reduces disease burden to improve CAR-T efficacy and safety
- Critical for aggressive lymphomas (e.g., DLBCL with elevated LDH or bulky disease)
Impact on Outcomes:
- Patients achieving CR/PR with bridging therapy had 42% lower risk of progression/death
- However, retrospective studies show bridging correlates with worse OS (48% 1-year OS vs 65% without)3, likely reflecting higher-risk patient selection
Commercial vs Academic CAR-T: Bridging Differences
| Factor | Commercial CAR-T | Academic CAR-T |
|---|---|---|
| Manufacturing Time | 30–41 days | 10–12 days (local production) |
| Bridging Use | 55–68% of patients | Less common (shorter wait times) |
| Delay Causes | Centralized facilities, insurance approvals, slot availability | On-site production avoids shipping delays |
| Toxicity | Higher CRS/ICANS rates (e.g., axi-cel) | Potentially lower due to fresher cells |
Key Drivers of Bridging in Commercial CAR-T:
- Extended Manufacturing Timelines:
• Median 41-day wait for liso-cel vs 30 days for axi-cel
• Centralized GMP facilities increase logistical complexity - Regulatory Requirements:
• Commercial trials often exclude high-risk patients needing urgent bridging
• Real-world use includes broader populations requiring disease control - Clinical Need:
• Patients with >1 extranodal site or CNS involvement often require bridging during delays
Balancing Risks and Benefits
- Effective Bridging (e.g., radiotherapy or RBP regimens):
• Achieves 65% ORR, reducing pre-infusion tumor burden
• Correlates with longer PFS (HR 0.58 for responders) - Risks:
• High-intensity bridging increases infection mortality (5/6 deaths in one study)
• No OS benefit observed despite improved response rates
Academic CAR-T platforms mitigate delays through rapid manufacturing, reducing reliance on bridging. However, commercial products maintain advantages in scalability and standardized quality control. The choice depends on disease kinetics, manufacturing access, and institutional capabilities.
In the video, Prof. Arnon Nagler from Israel speaks about commercial CVAR-T and Academic CAR-T.
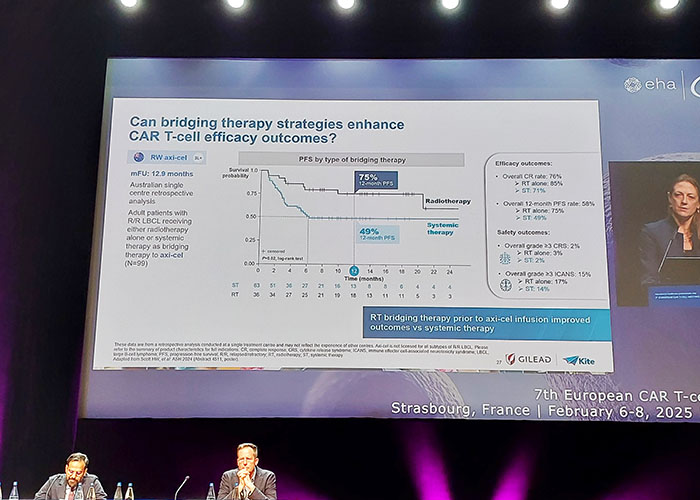
Photo: 7th European CAR-T conference. Strasbourg, France 2025.
Sources:
jitc.bmj.com
pmc.ncbi.nlm.nih.gov
chi.scholasticahq.com
pmc.ncbi.nlm.nih.gov
ashpublications.org
ashpublications.org
cancernetwork.com
jhoponline.com
Publication date: March 2025